KRUNAL ACID AGENCY - AHMEDABAD
- Home
- Krunal Acid Agency
- Chlorosulphonic Acid
Chlorosulphonic Acid
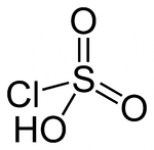
Chlorosulfuric acid is the inorganic compound with the formula HSO3Cl. It is also known as chlorosulfonic acid, being the sulfonic acid of chlorine. It is a distillable, colorless liquid that should be handled with care
properties
Chlorosulfuric acid is a tetrahedral molecule. The formula is more descriptively written SO2(OH)Cl, but HSO3Cl is traditional. It is an intermediate, chemically and conceptually, between sulfuryl chloride (SO2Cl2) and sulfuric acid (H2SO4). The compound is rarely obtained pure. Upon standing with excess sulfur trioxide, it decomposes to pyrosulfuryl chlorides:
2 ClSO3H + SO3 → H2SO4 + S2O5Cl2
Safety
ClSO3H reacts violently with water to yield sulfuric acid and HCl, commonly seen as vapors fuming from the liquid. Precautions, such as proper ventilation, associated with HCl should be observed.